Food safety is one of the chief concerns of all who work in the produce industry. The expectations and requirements for those who handle produce continue to proliferate and can be, at times, bewildering.
Under the Food Safety Modernization Act (FSMA), nearly all farm/ranch and food facility operators are required to implement food safety programs. Even small operations exempt from regulations are legally responsible for providing consumers with safe food. With all of the requirements coming from regulatory bodies and buyers, plus heightened food safety concerns from consumers, advocacy groups and others,
now is the time to take a closer look at the basic building blocks of a robust food safety program.
To better understand those building blocks, we will first review the following basic principles of food safety:
- Evaluate your risks.
- Prioritize your risks.
- Address each significant risk to minimize or eliminate that risk.
- Validate what you do to address each risk. Use available science.
- Document what you do. (If it isn’t written down, it didn’t happen.)
As we get started on this discussion of food safety building blocks, it is important to define some frequently used terms:
GAP
Good Agricultural Practices were originally outlined by the FDA in the 1998 publication
“Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables”. GAPs
are designed to identify potential pathogen contamination risks and suggest potential risk management practices.
GMP
Good Manufacturing Practices come from the Code of Federal Regulations 21 CFR110. GMPs identify contamination risks and risk management practices in packing, processing and holding operations.
SOP
Standard Operating Procedures. SOPs are written protocols for specific activities or tasks in an operation. An example of an SOP might be the written description of how to measure sanitizer concentration in wash water. The value of a written SOP is to ensure that critical practices are performed consistently.
SSOP
Sanitation Sanitary Operating Procedures. SSOPs are written protocols describing how
facilities and equipment are washed and sanitized. An SSOP includes a listing of the
cleaning and sanitation chemicals to be used, the appropriate concentration levels, the
methods to be followed, and how sanitation effectiveness is measured.
HACCP
Hazard Analysis Critical Control Point program. HACCP is a preventive system in which every step in the manufacturing, storage and distribution of a product is systematically evaluated for microbiological, physical and chemical hazards. An HACCP program is legally required in the fresh juice, meat, poultry, seafood and food canning industries.
HARPC
Hazard Analysis and Risk-Based Preventive Controls program; subpart C of the Current Good Manufacturing Practice, Hazard Analysis, and Risk-Based Preventive Controls for Human Food (the Preventive Controls Rule).
Like HACCP, HARPC is a mechanism to identify and manage microbiological, physical and chemical hazards. But unlike HACCP, HARPC addresses all significant hazards whether or not they are quantifiable. Instead of critical limits to control hazards at critical control points (CCPs), HARPC determines preventive measures to minimize or eliminate significant hazards at both CCPs and non-CCP areas. The hallmark of HARPC is a food safety plan including both a supply-chain program and a recall plan.
HAZARD
A biological, chemical or physical agent having the potential to cause illness or injury.
RISK
An estimate of the likely occurrence of a hazard.
VALIDATION
The process of evaluating scientific and technical evidence to prove that a control
measure(s) or the food safety plan as a whole, when properly implemented, can effectively manage the identified hazards.
VERIFICATION
The process of determining whether a control measure, or combination of control measures, is operating as intended.

How do I perform a risk assessment?
A comprehensive risk assessment is fundamental to understanding the risks of your operation. While your risks may be the same as or similar to other operations in your industry sector, some will be unique to you.
In addition, performing a risk assessment is a powerful educational tool that helps managers better understand the risks inherent in your operations—and the importance of addressing them. For these reasons, it is important that your team perform a risk assessment. Do not rely on outside consultants to do it for you, even though they may be working with, and supporting, your team’s efforts.
To perform a comprehensive risk assessment, start by assembling a team. Members might come from operations, quality assurance, maintenance, senior management and/or any other staff that are appropriate. Including a consultant or employee familiar with food safety science may also be helpful.
Next, develop an accurate flow diagram. Include all unit operations, any inputs (such as chemicals, water and ice) and packaging. This can be done for farm operations as well as for harvesting, packing, cooling or processing operations. After confirming that the flow diagram is an accurate reflection of your actual processes, number each step in the process. For example, “Receiving” might be #1, “QA Evaluation” might be #2, and so forth.
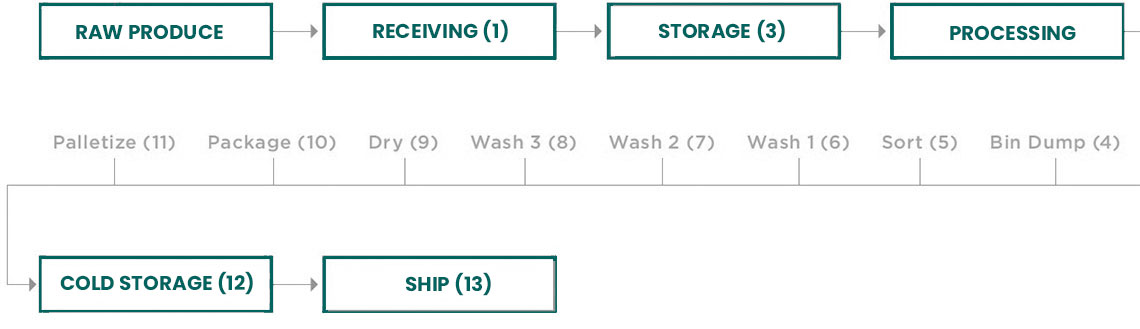
After numbering each step, brainstorm with your team and list all of the physical, chemical and biological hazards that could occur at each process step. For example, a biological hazard might be contamination by a human pathogen or insect. A chemical hazard could include excess sanitizing agents or agricultural chemicals not labeled for that product. A physical hazard might be pieces of glass or small stones, dirt or materials that could cause injury if consumed. The easiest way to perform this evaluation is to develop a simple table. List the potential hazards across the top, then list the steps in your process in the first column, as shown below:
| Process Step | Biological | Physical | Chemical |
|---|---|---|---|
|
#1 Receiving |
Salmonella from raw product contaminated in the field Insect parts Mold on fruit |
Rocks Wood silvers from pallets |
Unregistered pesticide residues |
|
#2 QA Evaluation |
Human Pathogens from hands of inspectors |
-- | -- |
|
#3 Dumping onto conveyor |
Cross-contamination from conveyor |
Plastic fragments from conveyor parts |
Residue from cleaning chemicals |
After potential hazards have been discussed and described for every process or handling step, evaluate which are reasonably likely to occur without preventive controls. This phase is called the risk assessment; risk being the probability that a hazard will occur. For example, on a farm the team may identify flooding as a potential hazard. But if that farm is not in a flood zone, and historically has not experienced flooding, the team may decide that the risk is not significant, and does not need further consideration.
After identifying significant hazards, the team then determines which preventive controls can manage or reduce that risk. In other words, for every significant risk there must be a program or activity implemented to reduce or eliminate it.
PRODUCT STEP
1. Receiving
| Potential Hazard | Significant? | Justification | Control Measures to Address Hazard | Responsible Person |
|---|---|---|---|---|
|
Wood Shards (P) |
Yes |
Has caused injury before |
Visual inspection by harvest crew, |
Harvest crew chief, QA |
|
Metal Fragments (P) |
Yes |
Has caused injury before |
Metal detector |
QA |
| Dirt / Soil |
No |
No history of food safety problems |
-- |
-- |
|
Pathogens on raw product (B) |
Yes |
Has been a problem on many produce products |
Evidence of GAPs operated by all |
Supply chain coordinator- |
|
Bacterial growth due to warming (B) |
Yes |
Bacterial pathogens can grow above ~38F |
Check temps upon arrival |
QA |
|
Pathogens from raw product on bins (B) |
Yes |
Bins can harbor pathogens |
Bins washed and sanitized on regular |
Sanitation |
Biol = B, Chem = C, Phys = P
For example, there may be a significant risk that raw produce could arrive already contaminated with Salmonella. If so, the team might require all suppliers of those products to demonstrate that they have audited GAP programs to minimize the risk.
Or, if the team has identified a significant risk of cross-contamination during washing, implement a wash water sanitation program. Most risks will be addressed through what HACCP plans have typically called prerequisite programs. Although HARPC does not use this terminology, the FSMA includes many of the same components as traditional prerequisite programs—GAP, GMP, cleaning, sanitation, worker training, and general hygiene requirements. These requirements will be described in more detail later on.
How do I develop or evaluate a GAP program?
Good agricultural practices, or GAPs, are a series of on-farm activities that address the main areas of concern where contamination may occur. Those areas have been outlined by the FDA in their 1998 publication “Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables” (the GAP Guide). They were codified in Title 21, Part 112—“Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption” (the Produce Safety Rule) under the FSMA.
Although not legally binding like the Produce Safety Rule, the GAP Guide contains more details for developing food safety programs than the Rule does. University extension programs and the FDA have developed and made available many other supplementary materials describing GAPs. In general, however, these guidance documents tend to be broad, and focus on areas of concern rather than specific actions.
For some commodities, such as leafy greens, melons, tomatoes, citrus, herbs, green onions, potatoes, mushrooms and others, there are specific documents that provide more detailed guidance. The USDA National Organic Program also provides more details. However, performing a risk assessment as described above is an excellent way to describe and address the risks for your commodities in your particular organization.
It is critically important that each operator of a farm, harvesting or packing operation, cooling facility, processing plant, distribution center or repacking operation takes the responsibility for assessing the risks in their operation. Your knowledge of operations, and how you handle products, will provide the most useful assessment. The guidance documents and Produce Safety Rule provisions can then provide such specifics as water quality standards, composting procedures and microbial standards, setback
distances from domestic animals, and others.
We have already referenced the FDA’s GAP Guide. In essence, most produce food safety standards, including the Produce Safety Rule requirements and commodity-specific guidance, have their roots in this original guidance document. In the FDA’s GAP Guide, the agency outlined eight specific principles, shown below:
BASIC PRINCIPLES OF GOOD AGRICULTURAL PRACTICES (GAPs)
- Prevention of microbial contamination of fresh produce is favored over reliance on
corrective actions once contamination has occurred. - To minimize microbial food safety hazards in fresh produce, growers or packers should use GAPs in those areas over which they have a degree of control while not increasing other risks to the food supply or the environment.
- Anything that comes in contact with fresh produce has the potential of contaminating it. For most foodborne pathogens associated with produce, the major source of contamination is associated with human or animal feces.
- Whenever water comes in contact with fresh produce, its source and quality dictate the potential for contamination.
- Practices using manure or municipal biosolid wastes should be closely managed to
minimize the potential for microbial contamination of fresh produce. - Worker hygiene and sanitation practices during production, harvesting, sorting, packing and transport play a critical role in minimizing the potential for microbial contamination of fresh produce.
- Follow all applicable local, state and federal laws and regulations, or corresponding
or similar laws, regulations or standards for operators outside the U.S. for agricultural practices. - Accountability at all levels of the agricultural environment (farm, packing facility,
distribution center and transport operation) is important to a successful food safety
program. There must be qualified personnel and effective monitoring to ensure that all elements of the program function correctly and to help track produce back through the distribution channels to the producer.
There is less control of the environment on a farm or ranch than in an enclosed packinghouse or processing facility. For this reason, some risks may be difficult to address directly. Remember that the GAP Guide and the Produce Safety Rule say that the operator should use GAPs in those areas over which they have a degree of control. Nevertheless, it is not acceptable for a food safety program to ignore a significant risk simply because it is difficult or impossible to control.

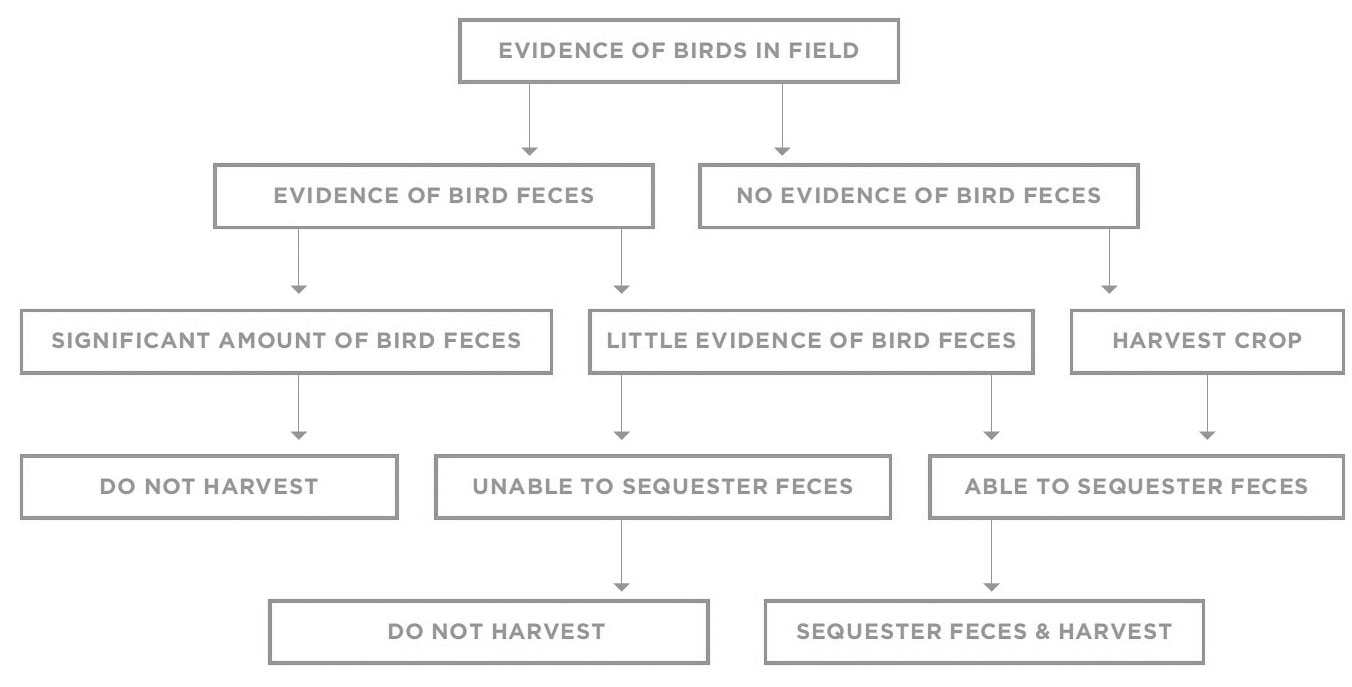
A common example of such a risk on farms and ranches is the presence of birds, some of which carry human pathogenic bacteria. Birds can and do enter fields, and it is often impossible to prevent that. But if birds are a significant risk, a reasonable GAP program would minimize bird attractants, such as in-field standing water and debris. It would also recommend against growing in areas close to where birds gather, such as landfills and wetlands.
Finally, develop a program of pre-harvest evaluation to determine the extent and seriousness of bird activity. Looking for bird feces can be used to construct a decision tree regarding whether to sequester an area of a field and not harvest the crop or to divert it from the fresh market.
Decision trees can be a very useful tool in GAP programs, as well as in other food safety programs. They allow the criteria for food safety decisions to be thought through in advance without regard to production and market concerns. An example of a simple decision tree for presence of bird activity in a field might be:
How do I develop or evaluate a GMP program?
|
|
|
Good Manufacturing Practices, or GMPs, are a series of rules regarding general cleanliness and hygiene for food handling facilities. GMPs, updated in 2015 as part of the FSMA, are published in the U.S. Federal Register (Code of Federal Regulations 21 CFR117) and have the force of law for off-farm packing operations and processors preparing ready-to-eat foods.
While harvest crews and on-farm packing and cooling operations are not subject to GMPs, provisions in the Produce Safety Rule are similar in nature and intent. In general terms, this statute mandates that food companies follow GMPs to assure the packing and processing environments are clean, and that no unacceptable substances enter the food product.
TOPICS ADDRESSED AS PART OF GMPs INCLUDE:
A summary of “GMPs for the 21st Century – Food Processing” can be found on the FDA website at: http://www.fda.gov/food/guidanceregulation/cgmp/ucm110877.htm
GMPs direct the operator to develop programs addressing:
- Personnel
- Plant and grounds
- Sanitary operations
- Sanitary facilities
- Equipment and utensils
- Processes and controls
- Warehousing and distribution
- Defect action levels
Within these categories, the GMPs describe activities referring to:
- Maintenance and sanitation processes
- Personal hygiene standards
- Water supply
- Pest control
- Plant design and construction
- Training
The following are some examples of GMPs referring to personnel:
- Management must take all reasonable measures and precautions to ensure that workers exhibiting symptoms of illness are excluded from operations where food could potentially be contaminated.
- All persons working in direct contact with food/food-contact surfaces must
conform to hygienic practices while on duty. - Outer garments must be suitable to the operation.
- Workers must maintain adequate cleanliness.
- Workers must wash their hands properly, and at appropriate times.
Examples of GMPs addressing buildings and facilities include:
- Grounds are kept in a condition that will protect against contamination of food.
- Areas in the facility’s vicinity that may provide harborage for pests are removed.
- Adequate drainage is provided to prevent breeding places for pests.
- Buildings and structure are suitable in size and design to facilitate maintenance
and sanitary operations. - Plants are constructed in such a manner as to prevent drip or condensate
from fixtures, ducts and pipes. - Adequate steps are taken to exclude pests, etc.
GMPs are lengthy and quite specific. You should become familiar with them, and incorporate those that apply to your facilities and operations. Most of these requirements are simply common sense, but they are comprehensive and may have been missed in your current food safety programs.
How do I develop SOPs for my operation?
Standard Operating Procedures (SOPs) are written instructions for performing standard tasks in a facility or on a farm. SOPs may cover any number of common activities, from pre-operation checklists to instructions for supervisors to check workers’ hands for cuts and lesions.
The SOPs remind everyone to perform necessary tasks. They also provide specific instructions as to what tasks to perform and how to perform them. A benefit of having written SOPs is that various workers can perform the tasks by following the SOP, even if they have not done so before. Another benefit is that the task is done consistently and does not change over time.
An SOP should address a specific task or series of tasks and the purpose of the tasks, it should specify who is responsible for the task, how often the task must be completed and what to do if the task was not or cannot be completed. An example of an SOP for entering a work area is:
SOP 1-01: Entering the Work Area
PURPOSE:
To ensure that all persons entering the work area have taken the necessary precautions to prevent foodborne pathogens from contaminating the product.
SUBJECTS:
All workers and visitors.
WHEN:
Whenever work is in progress and food products are present.
PROCEDURE:
- Only enter the work area if you are feeling healthy and well. Do not enter the work
area if you are coughing and/or sneezing, have an upset stomach, have an open and exposed cut or sore on your hands, or are otherwise feeling unwell. - Only enter the work area in clean shoes and clothing.
- Remove all jewelry and other objects that might fall into food, equipment or
containers. Only a plain wedding band is permitted. - Fix hairnet or other headgear.
- Wash hands thoroughly following proper hand-washing procedures.
- If applicable, put on smock or apron.
- Use a hand sanitizer.
- If applicable, put on clean gloves.
- Anytime your hands become contaminated, leave the work area and follow steps 1–8 before re-entering.
- Anytime you feel unwell, leave the work area. Do not return until you are feeling
healthy and well. Follow steps 1–8 before re-entering.
Such an SOP can be used to remind workers and supervisors to perform the tasks appropriate to ensuring the terms of the SOP. It can also be used as a checklist to document that the terms of the SOP have been completed. Constructing SOPs for common tasks helps ensure that they are completed correctly, consistently and in a timely manner.
How do I write and use an SSOP?
A Standard Sanitation Operating Procedure (SSOP) is an SOP specifically for cleaning and sanitation tasks. They address the pre-operational and operational procedures to prevent product contamination, and are a central building block of a rigorous food safety program.
Facility sanitation tasks are typically described as SSOPs. Each SSOP describes a specific task in the form of a detailed recipe for performing that task. The “recipe” should be detailed enough, and clear enough, so that a sanitation shift worker can follow the procedure and achieve a sanitary surface.
An SSOP should describe the task in detail from beginning to end. It should explain why that task is important and how often it must be completed. It should also list all tools and inputs needed to complete the task. An example of an SSOP is:
SANITATION OF CONVEYOR BELTS
GOAL:
Prevent buildup of organic matter and microorganisms on conveyors
so that they do not become a source of contamination.
IMPORTANCE:
Dirty conveyor belts can contaminate products that contact them.
FREQUENCY:
Monthly
TOOLS AND EQUIPMENT:
Potable water, hose, stiff plastic long-handled brush, soap, pressurized soap applicator, peroxide sanitizer, backpack sprayer, check sheet.
PROCEDURES:
- Remove belts from conveyors.
- Thoroughly rinse equipment, from top to bottom, with a pressure washer until all loose carrot sludge, bits and pieces have been removed.
- Mix soap as follows: ______________
- Apply soap with the pressurized soap sprayer.
- Scrub vigorously to remove small pieces of carrot and to remove invisible films.
- Rinse thoroughly a second time to remove all of the soap. The soap must be rinsed in order for the sanitizing solution (peroxide) to be effective.
- Reinstall the belts.
- Mix peroxide solution as follows: _______________
- All of the belts must be sprayed with the peroxide solution. The sanitizing solution is
applied with a backpack sprayer. - The Sanitation Crew Chief then inspects all sanitized surfaces.
- The Sanitation Crew Chief writes the time and date, and signs the sanitation log for
each individual belt. If any belt does not pass inspection, the Crew Chief notes that in the log and the crew must rewash and re-sanitize that belt until it passes inspection. - Replace belts.
After the task has been completed, the cleaned and sanitized surface can be swabbed and the swab sent to an appropriate lab for testing to verify the SSOP’s effectiveness. Swab tests, the most common method of conducting environmental monitoring, typically target indicator organisms such as Listeria species or environmental pathogens such as Listeria monocytogenes or Salmonella.
If the cleaning and sanitizing methods described in the SSOP are effective, the swab test result should show few or no viable bacteria on the swabbed surface. If, however, there are significant numbers of viable bacteria, then the SSOP is ineffective and sanitation methods need to be reevaluated and improved. In this way, all of the SSOPs can be improved and validated.
Having validated the SSOP, the sanitation team must follow its instructions, with confidence that the result will be sanitary surfaces. Such an approach of developing validated SSOPs for all cleaning and sanitation tasks results in a rigorous sanitation program that has been proven effective. The operator will thus have a high degree of confidence that the surfaces that have been cleaned and sanitized are, indeed, sanitary and reliably free of contaminants.
Another benefit of this approach is that it becomes unnecessary to constantly swab surfaces to verify that they are sanitary. The validated SSOP ensures that they are, as long as the sanitation crew faithfully follows the SSOP’s validated procedures. Revalidation of the SSOP is necessary if products, processes or procedures change.
In addition, periodic environmental monitoring as verification that the SSOP is still effective is important since microorganisms evolve and change their survival characteristics or may become resistant to the sanitizers in use. But for routine sanitation activities, swabbing frequency can be greatly reduced thereby
reducing costs.
Many operators develop rotation schedules based on the potential contamination risks presented by different pieces of equipment, or the frequency which a specific piece of equipment demonstrates measurable microbial levels after sanitation. This approach to risk-based sanitation verification can be effective in monitoring the operation’s sanitary status by targeting known problem areas and managing testing costs.
How do I develop a HARPC program?
A Hazard Analysis and Risk-Based Preventive Controls (HARPC) program, required for processing and off-farm packing facilities under the Preventive Controls Rule, is very similar to Hazard Analysis Critical Control Point programs (HACCP) which have been legally required for the fresh juice, meat, poultry, seafood and food canning industries for some time now.
HACCP was originally developed for the American space program to minimize foodborne illness incidents for astronauts while in space. Like HACCP, HARPC is designed to identify opportunities for food contamination with physical, chemical or biological contaminants, and to establish control measures to prevent that contamination from occurring.
Rather than testing finished products in an effort to detect contaminants, HARPC and HACCP programs seek to systematically prevent them. The heart of a HARPC program is a systematic hazard analysis and corresponding preventive control measures plan based on a comprehensive risk assessment, as described above. Based on the risk assessment, HARPC seeks to identify areas in packing or processing operations that, if controlled, will significantly minimize or eliminate contamination risks. The Preventive
Controls Rule’s provisions outline a comprehensive food safety program that includes HARPC and Good Manufacturing Practices (GMPs).
A food safety plan under HARPC includes seven components:
- A written hazard analysis
- Written preventive controls
- Written supply-chain program
- Written recall plan
- Written procedures for monitoring implementation of the preventive controls
- Written corrective actions
- Written verification procedures
How to conduct a hazard analysis was described above. Identifying and implementing preventive measures for hazards requiring preventive controls is the next step. Under FSMA, preventive controls include process controls, food allergen controls and sanitation controls. Preventive controls are procedures, practices or processes implemented for any activity or area in packing and processing operations that, when not controlled, may result in a significant hazard.
After identifying hazards requiring preventive controls, the team must establish how often each control will be monitored, and who will be responsible for ensuring their implementation. A preventive control may be implemented at a critical control point (CCP), or at areas other than CCPs.
A CCP has certain characteristics that limit its use to well-defined situations where it is possible to quantifiably monitor by measuring a set of parameters or limits (called critical limits under HACCP) around a process. An example of a CCP is a wash water sanitizer level that can be measured using a test kit or strip.
After determining preventive control measures, corrective actions must be established in case the process is not operating according to the food safety plan. An example of a corrective action would be running all products back through a recalibrated metal detector if the metal detector is found to be outside the critical limits of its calibration.
A preventive control must also be verified as appropriate to its “nature and role in the facility’s food safety system.” Verification involves checking the implementation and effectiveness of the preventive control. Is the preventive control being monitored as required? Is it achieving the desired results?
Finally, a record-keeping system must be established. All aspects of the food safety plan under HARPC must be documented. Those responsible for development and implementation of the plan, specifically the preventive-controls-qualified individual, must be properly trained to fulfill those responsibilities.

There is no minimum or maximum number of areas requiring preventive controls in any given operation. What is important is that all potential hazards are minimized or eliminated. Bulb-onion packing operations may have as few as a half-dozen reventive control measures in a perfectly adequate food safety plan. Examples of preventive controls may be metal detection and water sanitizer activity. Both are process controls that prevent significant hazards and happen to be measurable, and their operation can be documented.
When considering applying these principles to a farm operation, one can see immediately the difficulty in controlling naturally occurring hazards on farms. For example, while bird droppings in an orchard may present a hazard from the spread of E. coli O157:H7 or Salmonella spp., process control may not prevent that hazard.
This would also be true of Listeria monocytogenes cells in soil. Though they may represent a potential hazard, it would not be appropriate to establish soil as a hazard requiring a preventive control, since it is not practical to measure the spores in soil or to control them through any known process.
In fact, most agricultural hazards cannot, and should not, be prevented through an HARPC- or HACCPbased food safety plan. Instead, the use of GAPs has been identified by the FDA and the produce industry as a more appropriate way to address these hazards.
How do I validate my food safety processes?
An important aspect of preventive controls is their validation. Validation is based on collecting and evaluating scientific and technical information to determine if food safety processes, practices and procedures, when properly implemented, will effectively control the hazards.
Validation of food safety activities provides evidence to support your choice of preventive measures in order to ensure your program is doing what it is intended to do. In the absence of validation, we operate in an environment of assumption and hope, neither of which can be expected to be consistently effective.
Validation may be as simple as administering exams to workers as follow-up to food safety training, or it may involve measuring bacteria in wash water to show that the sanitizer activity is sufficient. Validation of cleaning and sanitation processes was discussed above in the context of performing micro-swabs of sanitized surfaces to demonstrate the efficacy of an SSOP.
In light of the recent foodborne illness outbreaks and recalls of fruit contaminated with Listeria monocytogenes, it is especially important to validate cleaning and sanitation processes to keep this robust pathogen out of your facility. Validation is based on research and performance measurements and so forms the scientific bedrock on which food safety activities are based. As such, it is a key element of a rigorous food safety program.
Remember, you cannot manage what you cannot measure. It is important to be able to measure the effectiveness of your program, and identify and change any instances where your preventive controls are ineffective.
Despite the best-laid plans, things can go wrong. This is as true in food safety as in any other walk of life. When a contamination event does happen, or may have happened, it is crucial to limit the extent of the damage or consequences as rapidly as possible.

The consequences are almost always economic but may also extend to public health. Speed and completeness of response are thus very important. You can’t limit the effects of a contamination event if you don’t know where the contamination came from or where it went.
In recent years, response teams from the FDA and other regulatory agencies have been very frustrated by opaque distribution chains within the produce industry that prevent them from rapidly determining the source and extent of contamination, and associated threat to public health. There has been a great deal of pressure on the produce industry to adopt clearer and more uniform traceback and traceforward procedures to facilitate emergency response.
Much progress has been made to this end, including an industry-wide effort called the Produce Traceability Initiative (or PTI). This initiative is a collaboration of several industry organizations and members of the industry to introduce a uniform case-level traceability program that will follow products from field-to-retail or foodservice. You can learn more about this initiative at http://www.producetraceability.org.
Whatever form of traceback program you adopt, you must be able to track all of your products one step back and one step forward. In other words, where did you get the product and where did you send it? This applies not only to fruits and vegetables but also to packaging materials and any other products that you ship, such as croutons, plastic forks and the like. In addition, you want to be able to trace and recover those products rapidly and efficiently.
The evolving industry standard is that you should be able to account for 99% of a shipped product within two hours. Many operations have automated this process and can complete it within minutes. What you do not want to do is to find yourself in the midst of a recall trying to figure out where your products came from or where they went. It is far better to have evaluated your traceability and recall systems in advance and performed mock recalls to demonstrate that they work seamlessly.
To prepare for a recall, start by assembling a crisis management team. Identify a recall coordinator and a recall team. The recall coordinator should be knowledgeable about every aspect of the company’s operation. Team members should include production, quality assurance, marketing/sales and public relations.
A recall organizational chart should be constructed whereby the primary and alternate individuals and both their work and cell phone numbers are listed so they can be contacted immediately when a situation arises. The recall coordinator and team would be in charge of determining the extent of the product withdrawal and releasing statements to the customers involved, governmental agencies, and press.
Establish a means of product tracking and customer identification.
The law already requires this, and there are many commercial and industry
resources to help structure the program. Then, test the program to make
sure that it functions smoothly, even under the worst circumstances. Recalls
don’t happen when they are convenient to you. They may well happen on
a Friday night, a Sunday morning or on Christmas Eve. Are you prepared to
respond at all times and under any circumstances? If not, you need to revisit
your traceability/recall program and make sure that it is robust. Remember,
a smooth and efficient trace-and-recall process may someday save your
company from ruin.

How can I use micro-testing to enhance my programs?
Over the past decade, there has been increasing pressure to test fresh fruits, vegetables and nuts for microorganisms. Much of this pressure has come from customers, especially large buying organizations. While testing for microorganisms can have a place in a food safety program, there are significant limitations to testing. You would do well to understand the power and the limitations of your proposed sampling and testing plans before embarking on a testing program.
While testing can yield useful information, it can also confuse, mislead and serve as a false basis for expensive decisions. The first thing to understand about testing for microorganisms on fresh produce or in produce environments is that you might find them. If you start looking for microorganisms, you had better have a plan of action in place, in case you find them.
There are several legitimate purposes for micro-testing of fresh produce. The first is because a customer requests or requires tests. A second is to detect the possible presence of pathogens. Another is to validate process elements through indicator organism detection. Yet another purpose is to model or understand a system’s microbiological ecology.
Finally, microbial testing can be used to track the fate of indicator organisms to better understand the system’s microbial dynamics. Before embarking on a micro-testing program, it is essential to clearly define the purpose(s) of testing as the structure of the test will be informed by the testing goals.
The choice of targeted test organism will largely depend on testing goals. In the produce industry, most water testing relies on detection of an indicator organism, like generic E. coli, as a surrogate for fecal contamination. Since E. coli is thought to often be associated with animal or human feces, it is likely to be present in water containing fecal contamination.
While E. coli is not necessarily fecal in origin, and the presence of generic E. coli does not necessarily mean there are human pathogens present, it indicates a likelihood of their presence since most food- and water-borne pathogens are fecal in origin. Testing for individual pathogens may be more difficult and more expensive than testing for generic E. coli, and testing for a single pathogen might miss the presence of others. Hence, E. coli tends to be the test organism of choice.

In some cases, tests for total coliforms or for fecal coliforms will take the place of generic E. coli tests. Coliforms are a group of bacteria characterized by their cylindrical shape. Fecal coliforms, a subset of coliform bacteria, grow at temperatures found in warm-blooded animals. While coliforms and fecal coliforms may be fecal in origin, many are not. Many coliforms and some fecal coliforms are common inhabitants of agricultural soils, and are not associated with feces at any part of their life cycle. Therefore, testing for coliforms and/or fecal coliforms is not recommended since presence of these bacteria may not indicate fecal contamination—making test results ambiguous.
To validate the efficacy of a wash water sanitizing chemical, one might test for total plate count (TPC), also sometimes called aerobic plate count. This test enumerates aerobic bacterial populations that may be present in the water. Since an effective water sanitizer should kill most such bacteria on contact, the TPC should be at, or near, zero. If it is not, then the sanitizer is not exhibiting the desired efficacy. Similarly, tests to validate the efficacy of environmental sanitizers usually test for TPC.
Some restaurant chains or other foodservice providers are asking produce suppliers to test for specific pathogens to keep contaminated produce lots out of the supply chain. In most cases, they request tests for Salmonella (of which there are thousands of different forms, or serotypes) and for E. coli O157:H7, a very virulent pathogenic form of E. coli infrequently found on lettuce, spinach, basil and other leafy vegetables.
Salmonella has been a problem on tomatoes, melons, mangoes, sprouts and many other products. Since these two pathogens have accounted for a large proportion of foodborne illness outbreaks associated with produce, they are the objects of most pathogen testing.
Finally, there are environmental pathogens—defined by the FDA as pathogens capable of surviving and persisting within manufacturing, processing, packing and holding environments such that food may be contaminated and result in foodborne illness if consumed. There is also the special case of the environmental pathogen, Listeria monocytogenes (L. monocytogenes).
Since it often becomes established in handling and processing facilities, many facilities perform L. monocytogenes-specific tests. L. monocytogenes, a natural inhabitant of soils, can survive for long periods and grow in soil and on plant material within a broad range of pH and temperatures. Once established in a processing plant, it is difficult to eradicate. It survives primarily in cool and wet areas such as drains, pipes, refrigeration units, and areas where condensate forms and collects. Since Listeria may be repeatedly introduced into processing facilities via incoming raw produce, rigorous sanitation of those areas is necessary.
L. monocytogenes may not cause major changes in the produce that a consumer could detect. So there is particular concern about the virulence (ability to cause disease) of L. monocytogenes in susceptible populations. When consumed in low numbers, L. monocytogenes can cause illness and even death in infants, pregnant women and immune-compromised individuals. Thus, most processing facility operators sample and test areas that may harbor Listeria species to verify that their sanitation processes are effectively excluding it. In the Preventive Controls Rule, the FDA refers to this type of testing as “environmental monitoring” and includes it as a means to meet verification requirements under HARPC.
So what is the problem with testing for microorganisms? None at all, if your testing program is meant to verify effectiveness of, or validate, a preventive control measure or process such as wash water sanitation or cleaning/sanitizing procedures.
Problems arise when looking for specific microorganisms, such as human pathogens, that are typically not uniformly distributed on produce or in the environment. They may be found in some parts of a field, but not in others, or on some individual fruit or vegetable and not on others. This makes sampling and testing difficult and complicated.
The probability of finding what you are looking for (if it is present), depends largely on the ability to test enough samples to find it. In the case of human pathogens on raw products in the field, or on finished products in a packing or processing facility, they are usually not present at all. If they are present, they may be unevenly distributed and present in low numbers. This makes them difficult to find, even when using the most sensitive of tests.
If, for example, Salmonella occurs on one melon out of every hundred, it would take a lot of test samples to detect it. This is the central problem of pathogen testing. While there are very fast, very sensitive test methods that can detect pathogens, the number of samples required may be so great that testing is impractical.
The difficulty of developing a sampling program that could reliably detect low-level pathogen contamination has been likened to finding a needle in a haystack. But it can also be described statistically. If a pathogen is present on produce or in the produce environment (a rare event), it may very well be present only sporadically and at a low frequency.
Years of testing by many companies and agencies show this to be generally so. The International Commission on the Microbiological Criteria for Foods points out that if you were to take 60 samples from a lot that was known to be contaminated at the 0.5% level, you would have a 74% probability of accepting this contaminated lot—and not detect the contamination.
Let’s take a look at this example below:
Probability of Accepting a Defective Lot with Indicated Proportion of Defective Sample Units
Number of Samples
| % Contaminated | 15 | 30 | 60 | 100 |
| 0.1 | 0.99 | 0.97 | 0.94 | 0.90 |
| 0.5 | 0.93 | 0.86 | 0.74 | 0.61 |
| 1 | 0.86 | 0.74 | 0.55 |
0.37 |
| 2 | 0.74 | 0.55 | 0.30 | 0.13 |
| 5 | 0.46 | 0.21 | 0.05 | 0.01 |
DEFECT LEVEL: 0.5%
SAMPLE UNITS TESTED: 60
Analysis: There is a 74% probability that all 60 samples will be found negative, and the lot will be accepted. So even after testing 60 samples from the lot, there is only a 26% probability that the pathogen will actually be found. A sample size of 100 would still miss it 61% of the time.
This simple statistical argument highlights the pitfalls of pathogen testing. You will probably not find it, even in the rare cases when it is there. If you don’t find it, you may believe that the product is safe when it is not. Therefore, testing for pathogens is an expensive and generally ineffective way to assure the safety of fresh fruits and vegetables.
Before embarking on any testing program, you must understand the implications of the results and have a plan of action, in case results are undesirable. In the case of pathogen testing, all products should be held so that they can be destroyed if a pathogen is detected.
Water tests should be conducted prior to the use of that water so that alternative sources, or alternative management schemes, can be employed if the water does not meet the microbiological specification. Sanitation chemicals and processes should be validated in advance, in case they are shown to be ineffective under some environmental conditions. Whatever the test, the operator should always have a plan of action before testing.
How do I implement an effective sanitation program?
With recent occurrences of foodborne illness outbreaks attributed to Listeria monocytogenes, some of which were associated with contaminated produce, the FDA has paid close attention to this particular environmental pathogen. As previously noted, Listeria, as an environmental pathogen, may be repeatedly introduced into cooling, packing, processing, storage and distribution facilities via incoming raw produce, equipment and foot traffic.
To prevent transient Listeria from developing resident populations in the facility, rigorous sanitation of those areas where the bacteria is likely to survive is essential. This involves establishing a written sanitation program, training employees to consistently implement the program and verifying its effectiveness with environmental monitoring.
Both cleaning and sanitizing methods and agents play a critical role. Choosing effective cleaning and sanitizing agents depends on operation-specific factors, such as the materials used to construct the facility and equipment. For example, some sanitizing chemicals may be corrosive depending on the materials used to construct your plant and/or equipment.
To determine which agents are best for your operations, consult with your chemical supplier. Cleaning and sanitizing methods are more standardized, but may be unique to a particular chemical and/or piece of equipment.
Routine cleaning and sanitizing is critical to avoiding biofilm formation. Biofilms are especially difficult to eliminate once they have formed. They are a leading factor in turning transient environmental pathogens into resident bacterial populations within a facility.

For example, we considered an example of an SSOP for sanitizing conveyor belts. SSOPs should be developed for all your equipment and areas of your facility where environmental pathogens were determined to be a significant hazard. This includes all food-contact surfaces, as well as non-foodcontact surfaces that may serve as a reservoir for environmental pathogens such as L. monocytogenes. As noted earlier, Listeria prefer cool, wet environments such as drains, pipes, refrigeration units and other areas where condensate forms and collects. All these points and areas should be part of a sanitation program.
For most produce handling and processing companies, sanitation has been a part of their operations for decades or for newer companies, since their inception. However, verifying sanitation by testing for environmental pathogens (environmental monitoring) is much more uncommon. Environmental monitoring is one of several activities the Preventive Controls Rule authorizes for verifying the effectiveness of a sanitation program.
If you are doing your own sampling for environmental monitoring purposes, it is important to develop an appropriate plan, including when to sample (before and/or after cleaning), sampling frequency and sampling location as well as training on the proper techniques for employees who do the sampling. Sampling plans should be written as an SOP so sample collection is consistently conducted.
For processing facilities and off-farm packing facilities, the Preventive Controls Rule stipulates that the number and location of sampling sites, and the timing and frequency of collecting and testing samples, “must be adequate to determine whether preventive controls are effective.” The rule also requires that environmental monitoring be scientifically valid and that operators identify the test microorganisms, the test methods used, the laboratory conducting the tests and any corrective actions taken due to the test results.
The FDA and other industry experts recommend that operators first screen test samples for Listeria species, since these tests are generally more rapid. Then test for Listeria monocytogenes as a follow-up for any positive Listeria screen tests. Initially, it may be good to test samples individually, but when your sanitation program has been validated and you are using testing as a verification activity, combining multiple samples from a given area into a composite sample is an effective alternative.
Composite samples allow you to sample more areas without added testing costs while still allowing for follow-up individual testing if a test result comes back positive. In the beginning, you may want to sample before and after cleaning to get a sense of the efficacy of your cleaning and sanitation practices. This information is especially informative to show your cleaning crews the tangible results of their efforts.
After your SSOPs are validated, and you consistently achieve your performance target, you may decide to conduct your environmental monitoring less frequently. It is recommended, however, to increase environmental monitoring activities when changes are made to cleaning personnel, equipment, cleaning processes or other parts of the SSOP.
There is no set formula for calculating the number of samples that are “adequate” to determine whether your controls are effective. Again, it comes down to the fact that you know your operations better than anyone else. You certainly may want to consult experts for advice, but ultimately you need to evaluate your plant, product and processes to determine the amount of sampling that gives you confidence that your sanitation program is effective.
Many trade associations, consulting companies and laboratories that process environmental monitoring samples have staff who are experts on developing sampling plans, and who conduct sample collection training. These and other resources are readily available if you are developing or revising your monitoring programs.
What can I do about all the audits I have to endure?
Audits are a way of life in the produce industry. They are not going away any time soon. While there have been numerous efforts to harmonize audits to reduce their number, many operators still submit to multiple, possibly redundant, audits. It is expensive and time-consuming to prepare individually for each audit, but different audits, and different auditors, have different requirements.
There is no easy solution to this problem. For now, the best you can do is to have a comprehensive, robust food safety program and complete, well-organized documentation to back it up.
This paper has argued that the heart of a robust food safety program is a systematic hazard analysis. A program based on such an analysis, with specific validated activities that address the significant risks, will stand up to any audit. You may not get a perfect score on them all, but you should have little trouble demonstrating the rational basis for what you are doing. With such a program as a base, you need only specifically prepare for the idiosyncrasies of the individual audit, a relatively simple process.
It is important to remember audits are only a measuring tool. Often, operators equate “passing an audit” with a comprehensive food safety program. This paper has shown that a comprehensive food safety program is composed of many foundational programs, with risk assessment as a central theme, to address the risk profile of your specific operation. An audit is a toll that permits you and your customers to assess your adherence to your program.
How can I train my staff to operate all these food safety programs?
The best food safety program will be of little value if it is not implemented properly. Monitoring and verification activities and documentation must all be done correctly. Worker health and hygiene is essential for food safety. These goals can only be achieved through effective and frequent training. Most workers want to do the right thing, but they need guidance.
Teaching general principles is important. All workers need to understand the importance of personal hygiene, proper handwashing and not coming to work sick. If you want workers to perform specific activities, they need to be taught to perform them properly.

Remember also that different people learn differently. Line-workers and field-workers are often poorly educated, and may even be functionally illiterate. Visual learning aids may be more effective than presenting text. Frequent short training sessions may be more effective than infrequent long sessions.
Most of all, training should be validated just like any other food safety activity. If you train workers to measure chlorine concentration in water, test them to validate that they have learned how to do it properly. If you train a crew to perform a sanitation activity, validate that they can do it correctly. In this way, training is no different than any other part of your food safety program.
What comes next?
The future of food safety in the produce industry.
The food safety landscape in the U.S. and globally is rapidly evolving, both from a technical and a regulatory standpoint. To paraphrase Yogi Berra, it is always dangerous to make predictions, especially about the future. The new U.S. food safety regulations have clearly had a tremendous impact, even though for many companies, compliance dates are still in the future. The Food Safety Modernization Act of 2011 provides the FDA with additional powers and additional responsibilities including:
- The power to order a recall, not just suggest one.
- The authority to inspect farming operations.
- Food facilities are required to register annually with the FDA. This provision does not
include farms, restaurants, retail food establishments or nonprofit food establishments in which food is prepared and served directly to consumers. - Registered food facilities are required to conduct hazard analyses and to develop and implement written preventive control plans. The plan must include: hazard analysis, preventive controls, monitoring, verification, corrective actions, and recordkeeping.
- Very small facilities and certain kinds of farm activities are exempt from some of the
requirements. Nevertheless, this paper strongly recommends that even exempt farms and facilities prepare a hazard analysis plan and substantially follow the regulatory requirements. It just makes good business sense to have the strongest food safety program as is practical. - Food importers are required to implement foreign supplier verification programs
and to take steps to verify that the food they import is safe. - Food facilities will be inspected with greater frequency and not less often than
once every five years. - The FDA is authorized to require that an article of food offered for import is accompanied by a safety certification from an accredited third-party auditor as an additional condition of granting admission.
What does this mean for the produce industry? It means that the FDA is going to expect most operations to have a hazard-analysis-based food safety plan and a workable, efficient recall plan.
The new regulations also place greater responsibility on facility operators to:
- Construct a rigorous food safety plan
- Be able to demonstrate its operation to the FDA
- Emphasize allergen controls and food defense plans
With some compliance dates already in effect, now is the time to review your food safety plans to make sure the essential building blocks for a robust, FSMA-compliant program are there and operating as planned.
References and Guidance Documents:
Guidance for Industry:
Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition (CFSAN), October 26, 1998. (https://www.fda.gov/food/guidance-documents-regulatory-information-topic-food-and-dietary-supplements/produce-plant-products-guidance-documents-regulatory-information
ProducePlantProducts/ucm064574.htm)
Code of federal Regulations 21CFR 112: Standards for growing, harvesting, packing and hold produce for human consumption
http://www.ecfr.gov/cgi-bin/text-idx?SID=1217844bf5d2c25e3c534fea3269628b&mc=true&node=pt21.2.112&rgn=div5
Code of federal Regulations 21CFR 117: FDA Good Manufacturing Practices:
http://www.ecfr.gov/cgi-bin/text-idx?SID=1217844bf5d2c25e3c534fea3269628b&mc=true&node=pt21.2.117&rgn=div5
Codex Alimentarius, 1997, FAO/WHO Food Standards:
http://www.fao.org/fao-who-codexalimentarius/en/
The California Leafy Greens Marketing Agreement (LGMA):
http://www.caleafygreens.ca.gov
Commodity-Specific Food Safety Guidelines for the Melon Supply Chain - 1st Edition:
https://www.fda.gov/downloads/Food/GuidanceRegulation/UCM168625.pdf
Commodity-Specific Food Safety Guidelines for the Fresh Tomato Supply Chain:
https://www.fda.gov/downloads/Food/GuidanceRegulation/UCM171708.pdf
Mushroom Good Agricultural Practices Program:
https://www.ams.usda.gov/services/auditing/gap-ghp/mushroom-gap
Florida Citrus Packers/Indian River Citrus League 2011 Food Safety Good Agricultural Practices:
http://ircitrusleague.org/wp-content/uploads/2012/02/food-safety-a-b-c-111107.pdf
Commodity-Specific Food Safety Guidelines for Fresh Culinary Herbs:
https://www.wga.com/resources/commodity-specific-food-safety-guidelines-fresh-culinary-herbs
Commodity-Specific Food Safety Guidelines for Green Onions:
https://www.fda.gov/downloads/Food/FoodSafety/Product-SpecificInformation/FruitsVegetablesJuices/GuidanceComplianceRegulatoryInformation/UCM203114.pdf
Commodity-Specific Food Safety Guidelines for Production, Harvest, Storing and Packing of Potatoes:
https://www.fda.gov/downloads/Food/UCM365582.pdf
University of California Good Agricultural Practices Program:
http://ucfoodsafety.ucdavis.edu/UC_Publications/UC_Good_Agricultural_Practices_GAP/
Cornell University Good Agricultural Practices Network for Education and Training:
https://gaps.cornell.edu/
University of Florida, IFAS, Sanitation, Food Safety and Security:
http://irrec.ifas.ufl.edu/postharvest/index/sanitation.shtml
The Produce Traceability Initiative:
www.producetraceability.org
Microorganisms in Foods 7 - Microbiological Testing in Food Safety Management, 2002 International Commission on Microbiological Specifications for Foods (ICMSF) Kluwer Academic / Plenum Publishers NY, NY.
About the Author

DEVON ZAGORY, PH.D.
President, Devon Zagory & Associates LLC
1-530-219-7489
devon@zagory.com
Devon Zagory has a PhD in Plant Pathology from the University of California, Berkeley, and has worked extensively as a consultant to the produce industry for more than 25 years. He is a past co-chair of the Technical Committee of the International Fresh-Cut Produce Association and was the Editor-in-Chief of the Third Edition of the IFPA Food Safety Guidelines for the Fresh-Cut Produce Industry. He has written numerous scientific publications and industry bulletins dealing with microbial safety, packaging, quality, long-distance shipping and operations. He has served as the Executive Director of the Center for Produce Safety at UC Davis and Senior Vice President for Food Safety & Quality Programs for NSF Agriculture, an NSF International company. Devon is currently the president of Devon Zagory & Associates LLC, providing services to the fresh produce industry in food safety, quality assurance, packaging, MA/CA and in international development programs.
